In an age where we care more about the ethics behind the products we use, we’re also forced to navigate the growing number of online marketplaces full of deals and dupes that stand in opposition to safe, clean makeup and skincare. Fake palettes and lipstick have been found to contain four to 15 times the amount of lead as their actual counterparts, according to a CBS News investigation. That $2 Kylie Lip Kit is guaranteed to do more harm than good for your health, in addition to “cost[ing] the industry about $75 million dollars,” as reported to Refinery 29 by Deborah Parker, Deputy Special Agent In Charge for Homeland Security.
There are many grey areas in the industry with the U.S. Food and Drug Administration, manufacturers, distributors and consumers all to blame. For starters, the only ingredient in cosmetics that need FDA approval are color additives.Besides that, they do not require “premarket notification, safety testing, review, or approval of the chemicals used in cosmetic products” according to a 2012 Congressional Research Service report. While the FDA may conduct inspections of cosmetic facilities, they do not require manufacturers to “file their product formulations” or register for any license to operate.
Many are calling for an overhaul of federal cosmetic regulations in light of the Johnson & Johnson lawsuits in which the corporation is being accused of their baby powder containing asbestos, leading consumers suffering from mesothelioma or ovarian cancer. In a statement to Vox, Johnson & Johnson claimed that they “have long supported legislation to modernize the US FDA’s regulatory authority… and believe this reform is essential to enabling the agency to increase their ability to protect the public.”
The problem is that the most recent legislation the FDA regulations follows is 80 years old. The Federal Food, Drug, and Cosmetic Act of 1938 has seen few amendments since it was set into motion. Luckily, a recent bipartisan bill is looking to change that: the Personal Care Products Safety Act.
Introduced in March by senators Susan Collins (R-Maine) and Dianne Feinstein (D-Calif.), provides stricter guidelines regarding substances that “may be deemed inappropriate for use in children’s products or only appropriate for use by professionals.” The act would also grant the FDA more authority over manufacturers, including requiring that they register with the FDA versus it being voluntary currently.
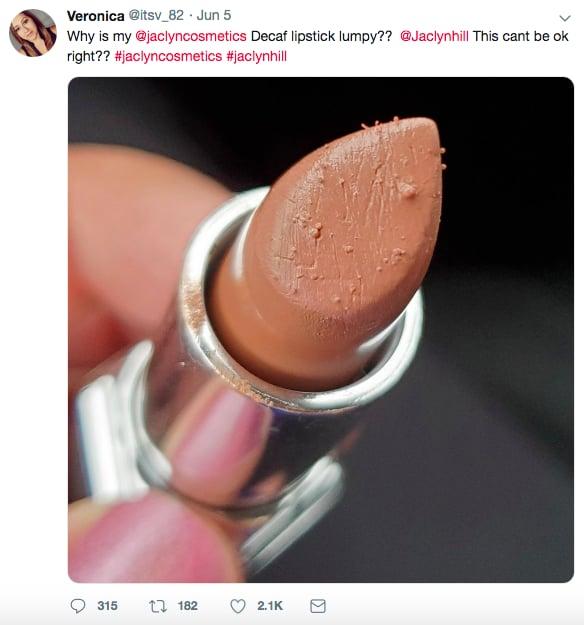
Trusted influencers are suffering the consequences of manufacturer error. YouTuber Jaclyn Hill launched a line of lipsticks; but upon arrival, fans found the tubes to be clearly contaminated. In her explanation video titled “My Lipsticks,” she explains that she had similar issues with another manufacturer several years prior and chose not to launch the brand.
The beauty industry leaves consumers to fend for themselves, leaving us no choice but to be more informed about the purchases we make. It’s become necessary to take the time to learn about a brand and read the reviews or suffer the consequences. No smokey eye look is worth stye.





![[Both photos courtesy of sonoma.edu]
Ming-Ting Mike Lee stepped in as the new SSU president following Sakakis resignation in July 2022](https://sonomastatestar.com/wp-content/uploads/2024/04/CC4520AB-22A7-41B2-9F6F-2A2D5F76A28C-1200x1200.jpeg)